Patrocinado
Mouse Kidney Fibroblasts: In Vitro Models to Understand Fibrosis
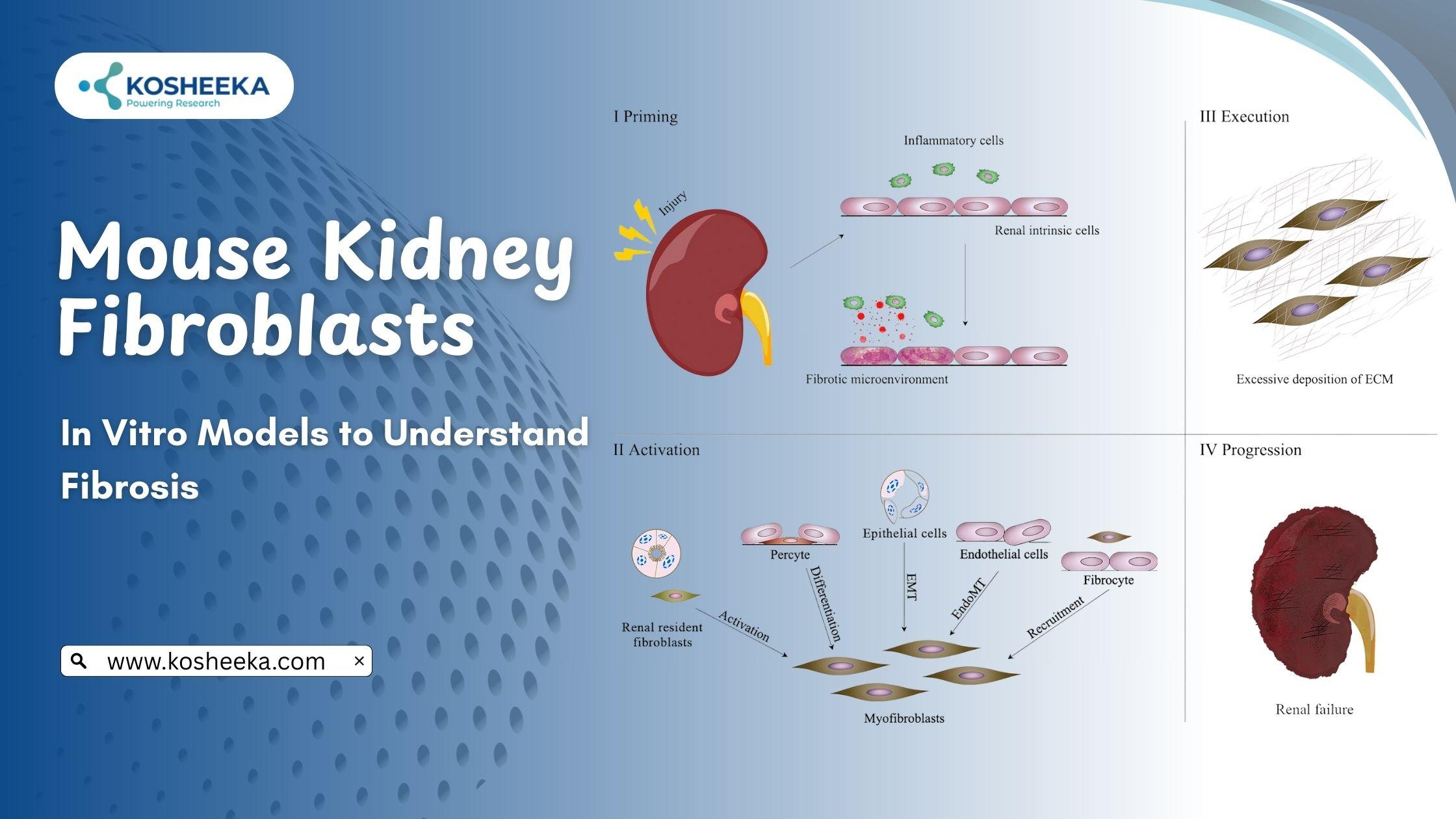
Mouse kidney fibroblasts are vital in vitro tools to study renal physiology and disease. These cells provide the structural support to the organ. However, their dysregulation results in fibrosis, a hallmark feature of chronic kidney disease (CKD). The absence of anti-fibrotic therapies and increasing morbidity and mortality due to CKD have accelerated research on these cells. They can offer valuable mechanistic insights into fibrosis and CKD progression. Such understanding can favor the identification of therapeutic targets for fibrosis and drug development.
Chronic Kidney Disease (CKD)
CKD affects approximately 10-14% of the population. It is marked by progressive loss of nephrons, vascular damage, inflammation, oxidative stress, etc. As a result, CKD leads to complete renal failure. Fibrosis is the primary contributor to CKD. It involves excessive deposition of extracellular matrix (ECM), which deteriorates the renal structure and subsequently its functions. The treatment modalities have a modest impact on CKD progression, while none target fibrosis. Researchers are attempting to understand this pathological process mediated by fibroblasts.
Kidney Fibroblasts
Fibroblasts are present throughout the body. They originate from Mesenchymal Stem Cells (MSCs) and assume a spindle morphology. The key function of these cells is to synthesize and secrete ECM proteins such as collagen. Additionally, they balance the levels of proteolytic enzymes and inhibitors of these enzymes to regulate the turnover of the ECM. Thus, they provide architectural support to the organ to maintain its functioning. During injury, fibroblasts transition into myofibroblasts to promote repair. However, the impairment of myofibroblasts results in fibrosis.
Myofibroblasts
Myofibroblasts are flattened cells with irregular morphology. They are terminally differentiated cells, distinguished from fibroblasts by their contractile property. These cells are rarely present in normal, non-pathological conditions. They have a characteristic expression of α-smooth muscle actin (α-SMA) and are morphologically and structurally similar to smooth muscles. Studies on mouse kidney fibroblasts have outlined different origins of myofibroblasts, including fibroblasts, endothelium, MSCs, pericytes, etc. However, establishing a precursor for myofibroblasts is still under investigation.
Fibrosis
Wound repair precedes the fibrosis event. In case of an injury to a nephron, myofibroblasts are activated. They secrete ECM proteins to repair the damaged tissue. The protein composition of this ECM is different from that of normal tissue. The collagen in this ECM elevates the stiffness of the organ. Myofibroblasts also drive ECM remodeling by regulating the levels of matrix metalloproteinases. The contractile property of these cells causes wound closure. In several wounds, myofibroblasts undergo apoptosis, while the injury site is repopulated by normal tissue cells.
Repetitive or severe injuries, however, dysregulate the entire repair process. Myofibroblasts persist and stimulate ECM accumulation. The increasing amount of ECM disrupting the normal tissue structure is referred to as fibrosis. It results in reduced blood flow and capacity for filtration. The continuous presence of myofibroblasts and the subsequent spread of ECM augment the structural loss of the tissue, leading to complete organ failure.
Signaling Pathways
The process of fibrosis is a complex interplay between fibroblasts and other cells. Kidney injury also triggers the recruitment of immune cells to clear damaged cells and participate in repair mechanisms. Immune cells also secrete factors for myofibroblast activation. Thus, inflammation often aggravates fibrosis. Knowledge about such pathways and their signaling by in vitro culture of Mouse Kidney Primary Cells can be translated into therapeutics. The following is the list of a few signaling pathways involved in fibrosis-
- TGF-β: TGF-β is the key driver of fibrosis. It simulates the development of myofibroblasts by epithelial-to-mesenchymal transition. These factors also mediate the influx of immune cells, which leads to inflammation. Other factors like TGF-β include FGF2, PDGFR, and EGFR. They utilize JAK/STAT, PI3/Akt, and MAPK pathways to directly or indirectly induce fibrosis.
- Development Pathways: Notch, Wnt, and hedgehog signaling pathways contribute to kidney development. Their expression is increased in CKD, indicating their involvement in fibrosis. Studies have revealed that these factors induce differentiation into myofibroblasts and immune cell activation.
- Ang II: Ang II is a component of the RAAS (renin-angiotensin-aldosterone system). It stimulates TGF-β, promotes inflammation, and causes vasoconstriction. Collectively, these mechanisms trigger hypoxia, which subsequently leads to oxidative stress and inflammation, exacerbating the disease.
Future Perspectives
Kidney Fibroblasts maintain tissue structure and homeostasis. However, their dysfunction leads to fibrosis- a key pathological process of CKD. In the absence of anti-fibrotic therapies, research on CKD treatment has shifted to studying mouse kidney primary cells and their involvement in fibrosis. Studies have delineated several pathways that can serve as targets for formulating more effective medications. TGF-β has entered clinical trials. Interventions that target Wnt pathways include α-Klotho, paricalcitol, and anti-DKK1 antibody. ACE inhibitors and ARBs target the RAAS pathways and have substantially improved eGFR rates.
These therapies underscore the significance of research on mouse kidney primary cells. However, it is crucial to understand that fibrosis involves multiple cells and pathways. Therefore, further details on the cell-cell interaction can provide more details about the process and potential therapeutic targets. To advance this research, Kosheeka delivers premium-quality primary mouse kidney fibroblasts tested stringently for their viability and purity.



