إعلان مُمول
Organoids Market Development: Challenges and Solutions for Scaling Up Organoid Manufacturing and Standardization Processes Globally
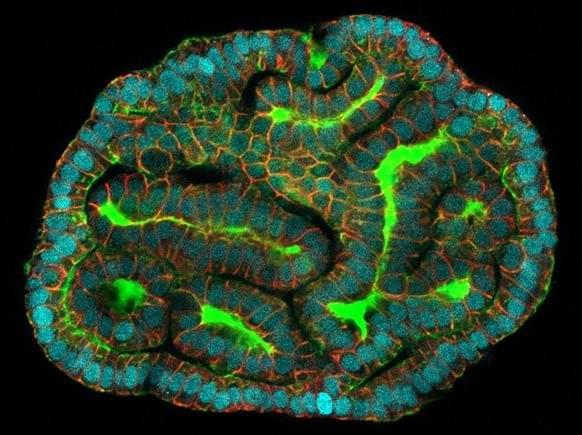
The organoids market is rapidly evolving, driven by increasing applications in drug discovery, disease modeling, and regenerative medicine. However, the transition from small-scale laboratory research to large-scale, commercial manufacturing poses significant challenges. Scaling up organoid production while ensuring standardization and quality is essential to meet growing global demand and accelerate market growth.
Key Challenges in Scaling Organoid Manufacturing
-
Complexity and Variability of Organoids
Organoids are complex, three-dimensional cellular structures that mimic human tissues, but their heterogeneity poses reproducibility challenges. Variations in cell sources, culture conditions, and differentiation protocols lead to batch-to-batch inconsistencies, impacting reliability for research and therapeutic applications. -
Manual and Labor-Intensive Processes
Current organoid production often relies on manual techniques that are time-consuming and prone to human error. This limits throughput and increases production costs, making large-scale manufacturing economically challenging. -
Lack of Standardized Protocols and Quality Control
There is no universally accepted standard for organoid cultivation, characterization, or quality assessment. This absence complicates regulatory approval processes and hampers widespread adoption in clinical and pharmaceutical settings. -
Regulatory and Ethical Considerations
Navigating regulatory requirements for organoid-based products is complex due to evolving guidelines and ethical concerns related to stem cell use and genetic manipulation. Ensuring compliance while scaling production adds an additional layer of difficulty.
Solutions Driving Market Development
Automation and Robotics Integration
Automation technologies, including robotic liquid handling and bioreactor systems, are increasingly being deployed to scale organoid manufacturing. These systems enable consistent cell seeding, media exchange, and environmental control with minimal human intervention, reducing variability and boosting throughput.
Automated imaging and AI-driven monitoring further enhance process control by providing real-time quality assessments, allowing early detection of culture deviations and ensuring uniformity.
Standardization Initiatives and Protocol Harmonization
Industry consortia, regulatory bodies, and academic groups are working to develop standardized protocols for organoid culture and characterization. Harmonized guidelines focusing on cell sourcing, differentiation markers, viability metrics, and functional assays will facilitate reproducibility and regulatory acceptance.
The establishment of quality control benchmarks and reference materials supports comparability across laboratories and manufacturing sites, accelerating global market integration.
Advanced Bioprocessing Technologies
Emerging bioprocessing platforms, such as microfluidic organoid-on-chip devices and 3D bioprinting, offer precise control over organoid architecture and microenvironment. These technologies improve scalability by enabling parallelized production with enhanced structural consistency.
Modular manufacturing approaches also allow flexible scaling tailored to specific organoid types and applications, optimizing resource use.
Regulatory Engagement and Ethical Frameworks
Proactive collaboration with regulatory agencies helps companies anticipate and meet evolving compliance requirements. Clear ethical frameworks and transparency in sourcing and manipulation of stem cells build trust with regulators, clinicians, and patients.
Industry-wide adoption of best practices in informed consent, data privacy, and biosafety ensures responsible development, paving the way for broader clinical adoption.
Market Impact and Growth Prospects
Overcoming scaling and standardization challenges will unlock substantial growth opportunities in the organoids market. Enhanced manufacturing capabilities enable high-throughput drug screening, personalized medicine, and cell-based therapies at competitive costs.
Global adoption will accelerate as reliable, scalable, and compliant organoid production platforms emerge, supporting demand from pharmaceutical companies, contract research organizations, and healthcare providers.
Conclusion
The organoids market development hinges on addressing critical challenges in scaling up manufacturing and achieving standardization. Through innovations in automation, protocol harmonization, advanced bioprocessing, and regulatory collaboration, the market is poised to transition from niche research to mainstream biomedical application. These solutions are essential for delivering consistent, high-quality organoids globally, driving the future of precision medicine and regenerative therapies.
الأقسام
إقرأ المزيد
Trekking in the rainy season brings a different kind of thrill. The smell of wet earth, misty mountain views, and the calm of the wilderness can make any trek feel magical. But there’s one thing that can quickly turn that magic into misery—getting drenched in your sleep. That’s where a trekking tent becomes your most trusted companion. In this blog, we’ll explore how to...

At Dsolutions, we’re more than just an ELV company—we’re your partner in delivering smart, reliable, and future-ready infrastructure. As the official Triax factory outlet in the UAE, we offer a comprehensive range of ELV systems, including IPTV, SMATV, CCTV, AV, networking, and automation solutions. Proudly serving Dubai and the wider Middle East, we bring top-tier ELV...



