Commandité
Amines - Important Alkyl and Aryl Substituted Organic Compounds
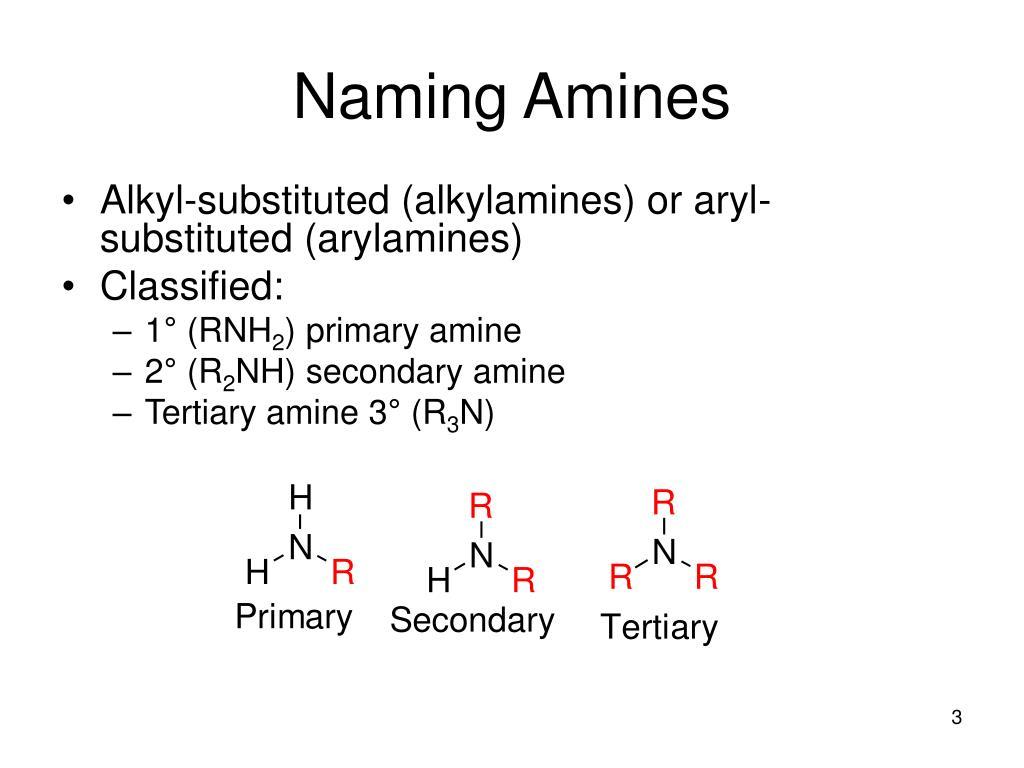
Amines are organic compounds that contain nitrogen as the key atom. The general structure of an amine involves an amino group attached to an alkyl or aryl group. Amino groups contain a nitrogen atom bonded to a hydrogen atom followed by a double bonded oxygen. Amines can be classified as primary, secondary or tertiary depending on the number of alkyl or aryl groups attached to the nitrogen atom.
Primary Amines
Primary amines contain one alkyl or aryl group attached to the nitrogen atom with two remaining hydrogens. An example of a primary amine is methylamine (CH3-NH2). Primary amines can further react to form secondary and tertiary amines. They are more reactive than secondary and tertiary amines due to the lone pair of electrons on the nitrogen available for reaction. Primary amines have a distinct ammoniacal odor and are combustible when exposed to heat or flames.
Secondary Amines
Secondary Amines contain two alkyl or aryl groups bonded to the nitrogen atom with one remaining hydrogen. An example is diethylamine (CH3CH2-NH-CH2CH3). Secondary amines are less reactive than primary amines, but more reactive than tertiary amines. They have higher boiling points than primary amines due to increased van der Waals forces between molecules arising from increased molecular weight. Secondary amines are colorless liquids or low melting solids.
Tertiary Amines
Tertiary amines have three alkyl or aryl groups attached to the nitrogen atom with no hydrogens. An example is trimethylamine . They are the least reactive of all amine types since the lone pair of electrons on the nitrogen is delocalized, decreasing its availability for reaction. Tertiary amines have higher boiling points than primary or secondary amines. They are typically colorless liquids though some may be waxy solids.
Preparation of Amines
There are several common methods used to synthesize amines in the laboratory and industrial settings. Reduction of nitro compounds is a widespread technique that employs reactions like catalytic hydrogenation to convert -NO2 groups to -NH2 groups. Gabriel synthesis involves the reaction of haloketones with potassium phthalimide followed by acid hydrolysis. Another approach is the Hoffman degradation which reacts alkyl bromides with aqueous ammonia at high temperatures. Aminolysis and hydrolysis of nitriles produces amines from organic nitriles. The Amadori rearrangement converts aldoximes to amines.
Properties and Reactions of Amines
Like other organic functional groups, the properties of amines are influenced by their structure. However, there are some general characteristics and reactions common to all amine subclasses.
Amines have a distinctive ammoniac-like odor detectable even at low concentrations. They readily dissolve in water due to hydrogen bonding between lone pair electrons on nitrogen and hydrogen atoms in water.
Amines react readily with acids to form water-soluble salts known as ammonium salts. With alkyl halides, they undergo nucleophilic substitution reactions facilitated by the lone pair on nitrogen.
Amines participate in reduction-oxidation reactions to transform nitrogen functional groups. They reduce aldehydes/ketones to form imines/enamines respectively in reductive amination reactions.
When reacted with acid chlorides or acid anhydrides, amines form amides through acid-base reactions. They also condense with 1 degree alcohols in the presence of acid catalysts to yield amines of higher molecular weight.
Amines react with electrophiles like isocyanates to form urea derivatives in important industrial reactions. Oxidation of amines produces imines, nitrones or nitro compounds depending on conditions employed.
Uses of Amines
Amines find extensive applications across many industries owing to their versatile chemical properties. They are important building blocks in the manufacturing of dyes, drugs, agrochemicals and various polymers.
In the rubber industry, secondary and tertiary amines demonstrate their value as accelerators that boost the vulcanization process. Primary amines act as blowing agents in the production of polyurethane foams for insulation/packaging.
Tertiary amines are commonly utilized as phase transfer catalysts to facilitate reactions between organic and inorganic substrates. Quaternary ammonium salts derived from amines exhibit antimicrobial properties.
Amines also impart basicity when incorporated into complex ligands for metal complexation. They allow intricate catalysis in industrial hydrogenations. Popular pharmaceuticals like local anesthetics and antihistamines exploit the physiological activities of certain amine groups.
amines represent a crucially important class of organic compounds with varied applications arising from their unique structural and chemical properties. Further advancements in green chemistry techniques are expected to expand the utility of these nitrogen-containing organic building blocks.
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)
Catégories
Lire la suite
Blaine Anthony Blaine Anthony is apparently perhaps of the most well known face in the open air television business. Blaine Anthony is commended for his true happy, his watchers frequently alluding to him as the keep going person on outside television keeping it genuine. He invests wholeheartedly in leftover his actual self and not buckling under industry strain to attempt to reclassify...

Manup Gummies Australia In the current quick world, the mission for internal agreement and flourishing has become more head than any time in ongoing memory. As we investigate the challenges of everyday presence, the allure of finding solace and tranquility has driven various to explore the universe of Man Up Gummies like. ✔️ Item Name — Manup Gummies Australia ✔️...



